New Sterility Assurance Strategies for Products Sensitive to Steriliza…
페이지 정보
작성자 최고관리자 댓글 0건 조회 4,013회 작성일 22-05-19 17:04본문
New Sterility Assurance Strategies for Products Sensitive to Sterilization Processes
By: Sopheak Srun and Molly Swanson
October 26, 2021
Categories: Sterilization Professionals
For a healthcare product to be considered “sterile,” the traditional options that have been accessible to a manufacturer are either terminal sterilization to a maximal sterility assurance level (SAL) of 10–6 or aseptic processing. While terminal sterilization is conducted on product after it is sealed in its sterile barrier packaging system, aseptic processing involves the handling of sterile components within a controlled environment, followed by the finished product being introduced into a sterilized package or container.
As the nature of microbial inactivation in a sterilization process is exponential, the probability of the survival of a microorganism can never be reduced to zero. Thus, the efficacy of a sterilization process is indicated in the SAL. Per ISO 11139:2018, SAL is defined as the probability of a single viable microorganism occurring on an item after sterilization; therefore, an SAL of 10–6 refers to a one in one-million chance that a single viable microorganism will be present after sterilization. As the numerical value of the SAL decreases (e.g., 10–7, 10–8, 10–9), the assurance of sterility increases. These concepts are illustrated in Figure 1 (from Figure 1 in ANSI/AAMI ST67:2019).
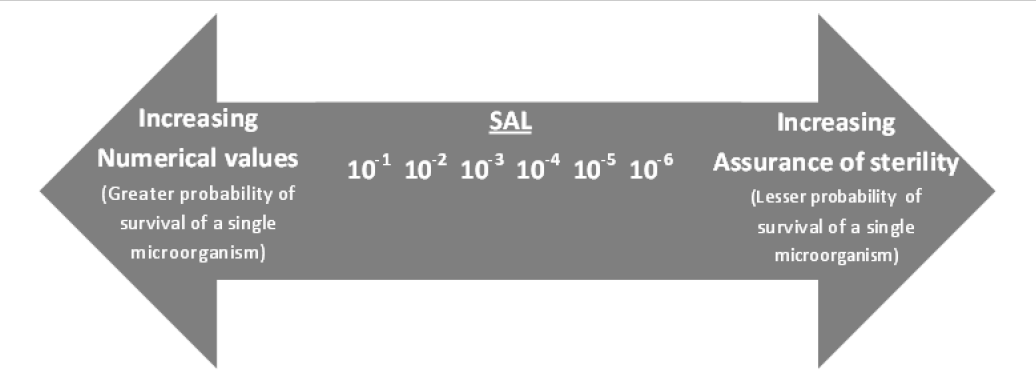 Figure 1. Numerical values of sterility assurance level (SAL) together with the concept of assurance of sterility. [Source: ANSI/AAMI ST67:2019 / ISO TS 19930:2017]
Figure 1. Numerical values of sterility assurance level (SAL) together with the concept of assurance of sterility. [Source: ANSI/AAMI ST67:2019 / ISO TS 19930:2017]
With the advent of new, innovative products that are sensitive to available sterilization processes (especially biologics and combination products), the need has increased in recent years for alternatives to an SAL of 10–6 or aseptic processing if a maximal SAL of 10 -6 cannot be achieved. With the publication of ISO/TS 19930:2017 and ST67 a framework now exists for how a manufacturer might perform a risk assessment to justify an alternative SAL as required by BE EN 556-1.
The introduction to the aseptic processing standard ANSI/AAMI/ISO 13408-1:2008/(R)2017 & A1:2013 states, “Wherever possible, healthcare products intended to be sterile should be sterilized in their final sealed container (terminal sterilization).” Implicitly, this is sterilization to a maximal SAL of 10 -6. ISO/TS 19930 gives aseptic processing equal weight compared with sterilization at alternative SALs (e.g., 10–5, 10–4, and 10–3), even though an alternative SAL would in many cases result in less risk to the patient compared with aseptic processing.
There is no doubt that properly validated and well-controlled aseptic manufacturing processes have produced safe and effective products that have served patients well for decades. However, compared with terminal sterilization, aseptic processing requires much more strict manufacturing process controls, training/expertise, and a significant quantity of tests of sterility as part of process simulation exercises, all of which can be prohibitive to many smaller companies.
The issue with comparing aseptic processing to terminal sterilization is that the difference in risk can be impossible to quantify. Per Lambert et al., one would need to test approximately three million product items (2,995,731) in order to demonstrate with 95% confidence that an aseptic process can achieve a probability of a nonsterile unit (PNSU) of 10–6, which more or less would be the equivalent of a 10–6 SAL for a terminal sterilization process.
Clearly, such a sampling plan would not be reasonable, and fortunately, it is not an expectation. Instead, process simulation sampling plans and sampling frequencies are determined based on risk. More typical sampling plans consist of 30,000 samples (c = 0 or c = 1), 15,000 samples (c = 0 or c = 1), 3,000 samples (c = 0), or 100 samples (c = 0), as described in 13408-1. Even with these large sample sizes, USP <1211> acknowledges that media fills and sterility testing are considered “analytically and statistically limited.”
The PNSU that could be demonstrated with 95% confidence from these process simulation sampling plans would at best be anywhere from 10–2 to 10–4. Although it certainly is possible that the actual PNSU is less than what could be demonstrated with these sampling plans, the exact PNSU in a given aseptic manufacturing process would be impossible to quantify.
Terminal Sterilization Following Aseptic Processing
To address these limitations, one concept described by Lambert et al. and in USP <1211> is to combine multiple processes (e.g., aseptic processing followed by terminal sterilization to a lower lethality level), which in combination could provide the same level of risk mitigation as a terminal sterilization process that is validated to achieve a maximal SAL of 10–6.
An example of how this might be achieved is described below. This hypothetical product is based on one of the examples provided in Annex D of ST67, but the example provided here goes a step further to show how aseptic processing could be used in combination with terminal sterilization as described in USP <1211>:
A device consists of an implantable drug solution in a delivery device, which injects precise amounts of the implantable drug solution to a target area. The delivery device is a syringe that is compatible with many sterilization modalities. The drug is a viscous solution; therefore, traditional sterilization methods such as gas, plasma, or vapor cannot be used. The drug solution, however, can withstand low doses of radiation.
As part of the manufacturing process, it is critical that the implantable drug solution be loaded into the delivery device. It is also ideal for the implantable drug solution to be provided in the delivery device to the end user. The drug solution is essential for the product to function and therefore cannot be altered. Further, the drug solution is heat sensitive above approximately 50°C. Due to the radiation and heat sensitivity of the drug component and the fact that it is viscous and cannot be sterilized through gas/plasma/vapor sterilization processes, no other sterilization processes could allow it to be sterilized to achieve a maximal SAL of 10–6.
In accordance with USP <1211>, the manufacturer has made a decision to terminally sterilize the delivery device and other associated components to a maximal SAL of 10–6, aseptically manufacture the drug solution, fill the delivery device, and then terminally sterilize the finished, packaged product to a lower lethality level (e.g., SAL of 10–3). Because of the risk that the product materials could support microbial growth, the manufacturer has also performed a study to demonstrate that any product bioburden (if present) would remain stable until the product is subjected to the sterilization process.
Compared with aseptic processing alone, one of the most significant advantages of this combination approach is a reduction in the overall risk to the patient. It is much easier to inactivate bioburden through terminal sterilization than to prevent microbial contamination during aseptic processing. This reduction in risk could allow for a reduction in the sample sizes and sampling frequencies used in the process simulation exercises required for aseptic processing. In some cases, could the need for media fills and sterility testing be eliminated altogether? This in turn could make aseptic processing a viable option for smaller companies, and it could allow for the introduction of new, innovative products that might not otherwise be feasible.
 Compared with aseptic processing alone, the combination of aseptic processing and terminal sterilization results in a lower risk to the patient. This combination approach has the potential to decrease the need for media fills and sterility testing considerably.
Compared with aseptic processing alone, the combination of aseptic processing and terminal sterilization results in a lower risk to the patient. This combination approach has the potential to decrease the need for media fills and sterility testing considerably.
This concept of combining aseptic processing with terminal sterilization to a lower lethality level is a fairly new concept that was introduced in March 2019 (USP41), and as such, it is not yet mentioned in standards and guidance documents (e.g, 19930, ST67). There are questions on how this could be implemented and what would be accepted by regulators and notified bodies. For example, would it even be necessary to document a risk assessment for justifying an alternative SAL? Would manufacturers need to obtain prior approval of this approach from regulators and notified bodies?
Combining Multiple Terminal Sterilization Modalities
Taking a step further, a manufacturer might find that their product could withstand sterilization exposures via different sterilization modalities, each to an alternative SAL, which in combination could provide a cumulative SAL of 10–6. Although such an approach is not explicitly allowed by the standards or by any national regulatory requirements, who is to say that such an approach would not provide an acceptable level of risk mitigation?
An example of how this might be achieved is described below. As with the previous example, this is an alternative approach that could be taken with one of the examples provided in Annex D of ST67:
A device contains a specialized low-friction polymer that helps reduce tissue damage during surgical procedures. The specialized polymer cannot withstand high doses of radiation, high temperatures, or other chemicals used in traditional sterilization modalities. This polymer cannot be altered, as it is a novel feature of the device and reduces tissue damage compared with existing devices on the market.
The device design is compatible with gaseous sterilization methods, and it has been found that the specialized polymer remains stable after a low temperature and short duration ethylene oxide (EO) cycle. However, cycle development studies have shown that the best SAL that can be achieved with EO is 10–3. Further, after performing product functionality studies, it has been found that the specialized polymer can withstand a low dose of radiation. Preliminary studies show the device can be radiation sterilized to a maximal SAL of 10–3.
With this information, the manufacturer performed a risk assessment per ST67 to determine if an alternative SAL is acceptable for the device. The assessment concluded that the risks associated with an alternative SAL (e.g., 10–3) outweighed the benefits of the device, and therefore, an alternative SAL is not acceptable. Because of this, the manufacturer has proposed to sterilize the device to a maximal SAL of 10–3 using EO, followed by sterilization with electron beam radiation to a maximal SAL of 10–3. This combination approach could provide a cumulative maximal SAL of 10–6.
Conclusion
In summary, the combination of aseptic processing and terminal sterilization results in a lower risk to the patient compared with aseptic processing alone. This approach could significantly reduce the need for media fills and sterility testing. Combining multiple terminal sterilization modalities could also be a viable option for some products. In addition, the combination approaches described in this article could potentially be used in lieu of performing a risk assessment to justify an alternative SAL.
To address the obstacles that many companies face in implementing these kinds of strategies, we are encouraging more collaboration within the industry, especially when this shared knowledge can improve efficiency and patient safety. Specifically, we urge those who have successfully implemented such strategies to share their experiences so that we all might benefit.
Sopheak Srun, MPH, SM(NRCM), CISS-EO, CISS-RAD, is a principal sterilization specialist at QTS, a Cretex Medical Company, in Bloomington, MN.
Molly Swanson, CISS-EO, CISS-RAD, is a sterilization specialist at QTS, a Cretex Medical Company, in Bloomington, MN.
- PrevNewly Arrived marketing news. 22.05.19
- NextKIMES (Korea Internation Medical Equipment Show) 2021 . 21.03.18
댓글목록
등록된 댓글이 없습니다.

